In previous article we saw what is the limits from chimney stacks for SO2 and NOx(measured as NO2). In this part we will see how to calculate the emissions of SO2 when we have measured sulfur contain in the liquid fuel. This computing method is based on stoichiometric understanding of process of fuel burn.
At first we should look at sulfur as independent compound in the fuel. When the burning process become with normal oxygen content – 3% we has this chemical equation:
S2 + 2O2 = 2SO2
Sulfur oxidizes to the sulfur dioxide. We have two sulfur atoms equivalent with molar mass 64 which react with two molecules of oxygen with summed molar mass 32+32=64. Then we are able to say:
64+2x32=128
So from one molecule of sulfur we received two molecule of sulfur dioxide with double increased molar mass and the correlation is 1:2. With this conclusion we could determinate how concentration of SO2 we should expect in chimney gases. Let start with 2 % sulfur contain in the fuel which we burn in combustion chamber. If we burn at least 700 kg per hour liquid fuel then we shall burn around 14 kilos of sulfur. From these 14 kilos we will receive 28 kilos of sulfur dioxide at this scheme:
700 kg x 2 % = 14 kg
1 mol S --> 2 mol SO2
14 mol S --> 28 mol SO2
14 kg --> 28 kg SO2
From 700 kilos of our 2 % contained sulfur powered fuel we had received a 28 kilos of sulfur dioxide. Now the questions are how chimney gases we should receive from this fuel. As far as I know 1000 kg of fuel give us 12 500 normal cubic meters of chimney gases. I have spoken with many peoples – all of them are engineers and many of them said that this digit is not so correct and it depends from type of fuel. It can decrease the size and gain the 20 000 m3. So I have been teach from my ex-boss on this value and we will work with it.
Before we start to work we will take care for dimensions. Because the limits are in mg/m3 we must change the dimensions in this 700 kg/h of fuel is a 0.7 tons/h of fuel. Our 28 kg/h of sulfur dioxide is a 28 000 g/h or 28 000 000 mg/h of sulfur dioxide – to describe this we could use the term: output of the emission.
And then:
From 0.7t/h we have 0.7 x 12 500 = 8 750 m3/h chimney gases,
The concentration of SO2 in chimney gases must be:
28 000 000 / 8 750 = 3 200 mg/m3
I have just prepared one table in google docs where everyone can calculate these emissions. If you know what is the load of your furnace for hour and what is the content of sulfur in the fuel you are able to calculate the emissions of SO2.
Little calculator.
In the end just want to say if you look careful over the formula you will see that there the limited factor is only sulfur contain in fuel. The quantity of burned fuel is given only for volume of chimney gases. In our example we break the sulfur dioxide level which is placed from EC in the
Air and water pollution control., FX Trading, Macroeconomics
Thursday 24 July 2008
Air pollution calculations part II
Tuesday 22 July 2008
Air pollution calculations part I
I am chemical engineer who is involved in water pollutions and air pollutions. Actually I am closely familiar with air pollutions and some of the methods of they calculation. Recently I have invited in a phone interview about my abilities in emission engineering. The people who lead the interview ask about some of the principles of calculating pollution of SO2 and NOx.
I had prepared my self before but may be not enough and two weeks ago they didn’t invite me to the face to face interview. But this is not the basis of this my post.
In this post I want to take a look about two methods of calculation of pollutions. At first I will describe the stoichiometric method which is based on the calculations of molecular masses of the each element in the chimney gasses. The second method is named Corinair from the CORe INventory of AIR Emissions. This methodology is developed from the then European Topic Centre on Air Emissions under contract to the European Environment Agency.
At first we should see what are the limitations in EU about some pollution compounds such as Sulfur Dioxide. The Directive 2001/80 describe those emission limits and give the specific differences between appliances depend from them heat power.
The Sulfur dioxide has a linear decrease chart where you could calculate the limit. This is only for liquid fuels and with 3% oxygen content burning of course and there is the chart: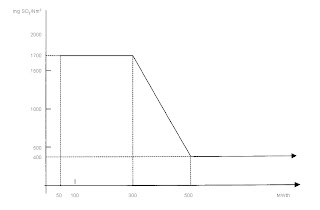
As you see here the limit of SO2 emissions is 1700 mg SO2 per normal cubic meter if your combustion plant has less than 300 MWt heating power. From 300 to 500 we have linear decreasing from 1700 down to 400 mg/Nm3. And for big combustion plants we have 400 mg/Nm3 for limit of SO2.
If the fuel is a gaseous fuel at general (it means natural gases not this which could be received in some refineries from different types of cracking processes) the limit of SO2 is 35 mg/Nm3.
For NOx the limits are quite different. 
So every of this info about limits you are able to see here.
Monday 7 July 2008
Weekend in Esen village.
Esen village is placed near the city of Karnobat and it is a small place with round 70 people all of them oldest than 65 years. Here in this small village people grow some animals like gooses, goats, hens, cows and ships. But the biggest interest of this entire people is the vineyards which are situated at 3 km near the village.
Actually my visit was related with spreading of pesticides against mildew of vineyard. Actually we started to do our job at 6:00AM and at 11:30 AM everything was done. As a fact this was not my first visiting. I have visited this village from 1999 year every year, but this time I took my photo and made some pictures which could be shown in my blog.
At first it is a panorama from the vineyard. This hills is the foot of Stara Planina mountain. The air is totally clean and the sky is blue :)
The second picture is Mayoralty of Esen village. The building is opposite to the basic square and of course of its back is the Health care service which is totally abandoned and ruined. Here doctor comes once time per weeks and if someone have any problems he write out some aspirin. 
The third my picture is The Church of the village. It is brand new building here and it has been building for two years. Now the church still not functional because there is not priest who will live here. Actually the church was built in the courtyard of the un-functioned school which is also abandoned and ruined. Here is the church: